About Equivalent Weight Calculator (Formula)
The Equivalent Weight Calculator is an important tool in chemistry, especially when dealing with reactions involving acids, bases, and salts. Equivalent weight helps in determining how much of a substance is needed to react with a certain amount of another substance. This guide will explain the formula for calculating equivalent weight, provide step-by-step instructions on how to use the calculator, and include examples and frequently asked questions to clarify any doubts.
Formula
The formula to calculate equivalent weight is:
Equivalent Weight (EW) = Molecular Weight (MW) / ΔE
In this formula, the molecular weight is expressed in grams per mole, and ΔE represents the number of moles of reactive species per mole of the substance.
How to Use
- Determine the Molecular Weight: First, find the molecular weight of the substance you are working with. This can be found on the periodic table or through chemical databases.
- Identify ΔE: Identify the value of ΔE, which represents the number of moles of reactive species produced or consumed in the reaction.
- Apply the Formula: Substitute the values of molecular weight and ΔE into the formula to calculate the equivalent weight.
- Result: The resulting value will give you the equivalent weight of the substance in grams per equivalent.
Example
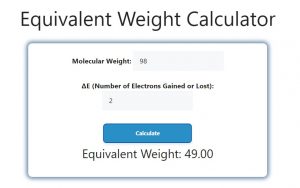
Suppose you have a substance with a molecular weight of 98 g/mol, and ΔE is 2. To find the equivalent weight, you would use the formula:
- Molecular Weight (MW) = 98 g/mol
- ΔE = 2
Now, substitute the values into the formula:
EW = 98 / 2
EW = 49 g/equivalent
Therefore, the equivalent weight of the substance would be 49 grams per equivalent.
FAQs
1. What is an equivalent weight calculator?
An equivalent weight calculator helps determine the equivalent weight of a substance based on its molecular weight and the number of reactive species.
2. Why is equivalent weight important?
Equivalent weight is important for stoichiometric calculations in chemical reactions, allowing for accurate measurement of reactants.
3. How do I find the molecular weight of a compound?
Molecular weight can be found by adding the atomic weights of all the atoms in a molecule, typically listed on the periodic table.
4. What does ΔE represent?
ΔE represents the number of moles of reactive species produced or consumed in the reaction involving the substance.
5. Can I use this calculator for all chemical compounds?
Yes, you can use the equivalent weight calculator for various chemical compounds, including acids, bases, and salts.
6. How does equivalent weight differ from molecular weight?
Molecular weight is the total weight of a molecule, while equivalent weight refers to the weight of a substance that will react with one mole of another substance.
7. Is equivalent weight the same as gram equivalent weight?
Yes, gram equivalent weight is another term used interchangeably with equivalent weight.
8. What happens if I use the wrong ΔE value?
Using the wrong ΔE value can lead to inaccurate calculations and affect the results of your chemical reactions.
9. How do I calculate ΔE for a reaction?
ΔE can often be found in the reaction stoichiometry; it indicates how many moles of a particular species are involved in the reaction.
10. Can equivalent weight be used in titration calculations?
Yes, equivalent weight is frequently used in titration calculations to determine the concentration of solutions.
11. How can I ensure accurate molecular weight calculations?
Use a reliable source, such as a periodic table or chemical database, and double-check your calculations.
12. Is equivalent weight used in any specific industries?
Yes, equivalent weight calculations are used in pharmaceuticals, chemical manufacturing, and environmental science.
13. Can I use this calculator for ionic compounds?
Yes, equivalent weight calculations can be applied to ionic compounds as well.
14. What is the significance of equivalent weight in acid-base reactions?
Equivalent weight helps determine how much acid or base is needed to completely neutralize a given amount of the other.
15. Is there a difference in equivalent weights for different reactions?
Yes, equivalent weights can differ depending on the type of reaction and the substances involved.
16. How do I convert equivalent weight to molarity?
To convert equivalent weight to molarity, use the equivalent weight to find the number of equivalents in a given volume and then calculate the concentration.
17. Can I find equivalent weight using molecular formulas?
Yes, equivalent weight can be calculated from the molecular formula by determining the molecular weight and ΔE.
18. Are there any online tools available for equivalent weight calculation?
Yes, various online calculators can assist with equivalent weight calculations, simplifying the process.
19. Can I apply equivalent weight calculations in environmental studies?
Absolutely! Equivalent weight is relevant in environmental chemistry, particularly in assessing pollutant reactions and interactions.
20. How often do I need to use equivalent weight calculations in chemistry?
Equivalent weight calculations are commonly used in many areas of chemistry, especially in stoichiometry and reaction planning.
Conclusion
The Equivalent Weight Calculator is a valuable tool for anyone working in the field of chemistry. By understanding the formula and following the outlined steps, you can accurately calculate the equivalent weight of various substances. This knowledge is essential for effective stoichiometric calculations in reactions involving acids, bases, and salts. Whether you are a student, researcher, or professional chemist, mastering equivalent weight calculations will enhance your understanding of chemical interactions and improve your overall effectiveness in the laboratory.
